The story behind the winning photos

The EAG would like to thank all who submitted photos to the Photo Contest 2015 and all who casted their vote. We have 2 winners: Ingrid Smet for the theme ‘Earth, Fire, Air, Water’, and Anton Bischoff for the theme ‘What is geochemistry for you?’. Here is the story behind the winning photos.
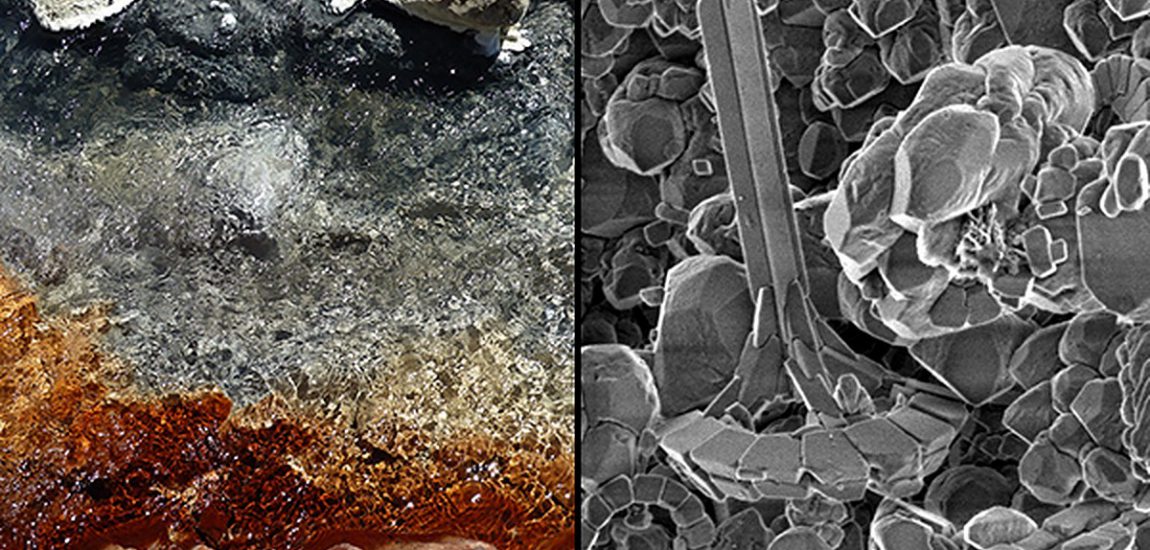
Theme ‘Earth, Fire, Air, Water’: The beauty of disequilibrium – The chemistry of rock destruction as it happens.
PhD fieldwork brought me to the Greek islands where I studied and sampled different volcanic deposits. I thereby felt that detailed photography of the outcrops and large-scale magmatic structures was important documentation of the ´bigger picture´. My fascination for both the large variety of volcanic phenomena and their geochemical origin has only grown since then. So whenever I get the chance to explore a new volcanic region, my camera is always within easy reach!
I took this particular picture during my August 2015 visit of Mount Papandayan, one of the many active arc volcanoes on the Indonesian island of Java. Whereas historical eruptions were all phreatic explosions, the volcano´s magmatic activity resumed since November 2002. The present-day complex stratovolcano has four large summit craters which contain active fumarole fields. So when the volcano´s early warning status allows visiting the area, it is a popular destination for those keen to explore hydrothermal phenomena such as bubbling mud pools, fumarole vents, sulphur deposits and small streams of mainly bluish water with milky white deposits zigzagging their way around. I was particularly intrigued by the zoned pattern that formed at the merge of differently coloured hydrothermal streams.
As I wanted to learn more about the geochemical story behind the phenomenon, I sent this photograph to Dr Richard Henley, an experienced researcher of hydrothermal systems. He kindly informed me that the differently coloured water layers represent fast hydrothermal erosion with geochemical separation of elements. Upon contact with air saturated groundwater, the volcanic sulphur oxidises and forms a sulphuric acid with pH less than 1. This acid solution reacts with the surrounding hydrothermal breccias to form kaolinitic clays, thereby dissolving the rock´s chemical components. FeO will quickly become pyrite (black in the image) which in turn oxidises to FeO(OH) (orange in image) and sulfate. More soluble elements such as Na, K, Ca, Mg and Al dissolve and are flushed away by the stream, but where the acid water wets the rocks above water level and evaporates, these elements are precipitated into alums and other salts (white in image). Silica initially also dissolves into the acid water but is precipitated upon changes in water temperature and pH (grey in image). The different processes separate the rock´s components and flush dissolved rock away – geology happening fast.
So the beauty is in how simple chemistry separates the elements one from another, with nothing being in equilibrium so that these processes self organise into the colourful pattern of the image.
About the author
Ingrid Smet received her PhD in Geology from Ghent University (Belgium) in 2014 for her geochemical and petrological research into the volcanic rocks from the western part of the South Aegean arc. Since 2015 she pursues her scientific interests as a voluntary researcher whilst doing free-lance work for Volcano-Adventures/ VolcanoDiscovery. Through the latter she can explore active volcanoes and share her passion for igneous geology with the general public; the former allows her to write up her PhD dissertation in scientific papers and to continue her research interest in the geochemistry and petrology of subduction zone magmatism, possible links between tectonics and volcanism, the microscopic study of igneous rocks, crustal differentiation processes, the connection between plutonic and volcanic rocks…

Theme 'What is geochemistry for you?': The Tower of Chalk
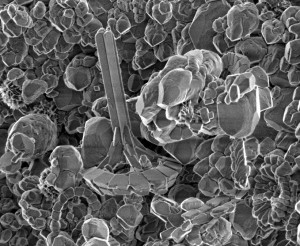
This image was taken with a scanning electron microscope at the University of Copenhagen in 2015. It was actually snapped during my training on the instrument, along with an expert professor and a fellow student. We were looking at a piece of chalk from a chalk pit in Aalborg, Denmark. This chalk is Maastrichtian, making it a little more than 65 million years old. Chalk is usually made up of remnants of coccoliths. Coccoliths are tiny calcite shields made by microscopic unicellular marine algae. The image shows a single shield (about 5 micrometers in diameter), but they are usually formed along with several others to envelope the algae in what is called a coccolithosphere. What is interesting about this specimen is its large “spine” formation, almost resembling the Eiffel Tower. Usually the coccoliths are simple flat discs, so we simply found this one to be really cool and snapped what turned out to be a really nice image of it. I chose to submit it to “what is geochemistry to you” because this was one of those things, that really exemplifies how much beauty and awe you can find in what is basically a grain of dust.
About the author
Anton Bischoff is a Masters student of Nanoscience at the University of Copenhagen. Currently working on his master thesis in the NanoGeoScience group at UCPH: A project dealing with mineral carbonation of silicate minerals, to bind CO2 as carbonate rock. This work will hopefully conclude with a Masters degree in Nanoscience in november of 2016.

